COMPLETE SYNTHESIS OF IDARUBICIN
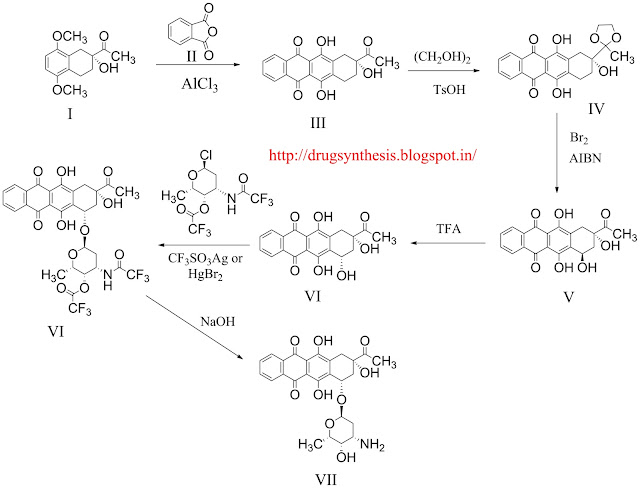
condensation of chiral tetraline (I) with phthalic anhydride (II) by means of AlCl3 at 180 C gives the naphthacenedione (III), acetyl group which is ketalized with ethylene glycol and p-toluenesulfonic acid yielding the dioxolane (IV). The hydroxylation of (IV) with Br2 and AIBN in CCl4/CHCl3 affords the 4-demethoxy-7-epidaunomycinone (V), which is isomerized with TFA yielding 4-demethoxydaunomycinone (VI) . The condensation of (VI) with the acylated hexopyranosyl chloride (VII) by means of CF3SO3Ag of Br2Hg affords the trifluoroacetylated 4-demethoxydaunomycin (VIII), which is finally deprotected by treated with NaOH to eliminate the trifluoroacetyl groups

Reference
Arcamone, F.; et al.; Synthesis and antitumour activity of new daunorubicin and adriamycin analogues. Experientia 1978, 34, 1255
Bernardi, L.; Arcamone, F.; Patelli, B.; Di Marco, A. (Pharmacia Corp.); Daunomycin analogues, their preparation and use. DE 2525633; US 4046878
tags-synthesis of NSC-256439, IMI-30, DMDR, Idamycin, Zavedos,HOW IDARUBICIN IS SYNTHESIZED

No comments:
Post a Comment